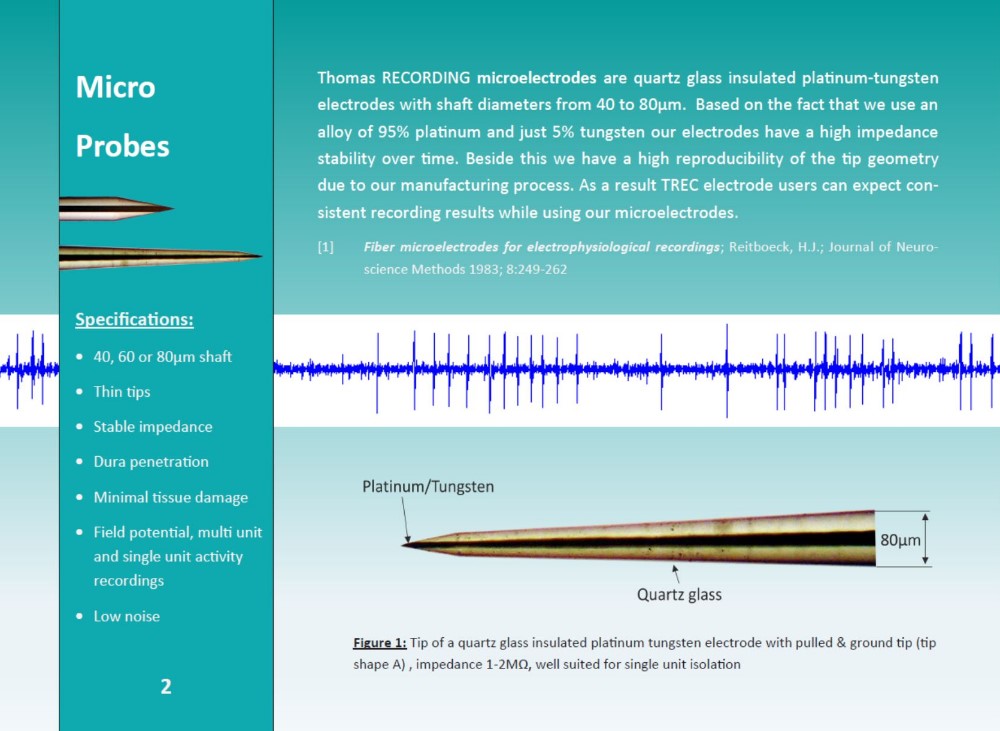
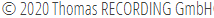(NEUROSCIENCE PRODUCTS)
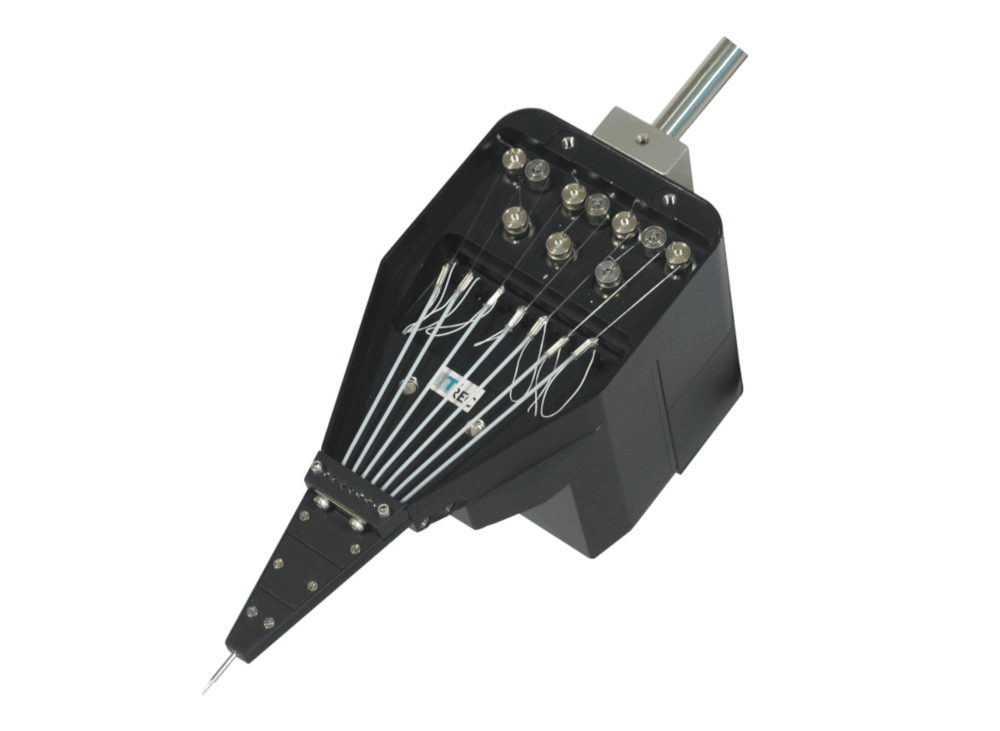
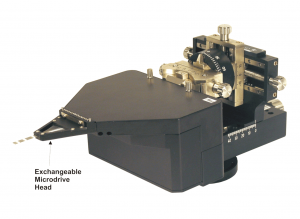
System Eckhorn Microdrive
7-Channel Single Electrode System
System Eckhorn
7-Channel Single Electrode System
.
The multielectrode manipulator “System Eckhorn” was developed in the laboratory of Prof. Dr. R. Eckhorn at the Department of Neurophysics, Philipps-University Marburg, Germany and successfully used for parallel independent insertion of fiber microelectrodes through the intact dura into the brain of chronically prepared cats and monkeys (see “downloads & publications”: [20],[21]).
The 7 electrode Eckhorn microdrive allows to drive up to seven fiber microelectrodes (diameter=80µm) independently from each other to different depths of the brain. The max. electrode travel distance of each fiber microelectrode is up to 35mm (electrode travel extensions are possible and available on request!). A 7 channel low noise preamplifier is integrated in the microdrive metal chassis. The microelectrodes are completely shielded by the microdrive chassis (working like a Faraday cage) so that electrical interference from the laboratory environment does not cause noise problems. Due to the rubber tube driving principle of the Eckhorn Matrix it is possible to record neural signals while the electrodes are moving in brain tissue!
Key features:
- Axial resolution better than 1μm, x-y-Positioning with a grid and special holder
- Patented rubber-tube drive, no hysteresis, slick or free motion due to patented rubber tube drive (avoids drawbacks of cable, direct or hydraulic driven systems)
- Electrode travel range up to 35.000μm
- Variable speed range from 0…250μm/s, higher velocity on request
- 7- and 16- channel Version available
- 28 or 64 channel Tetrode Versions available
- 49 or 112 channel Heptode Versions available
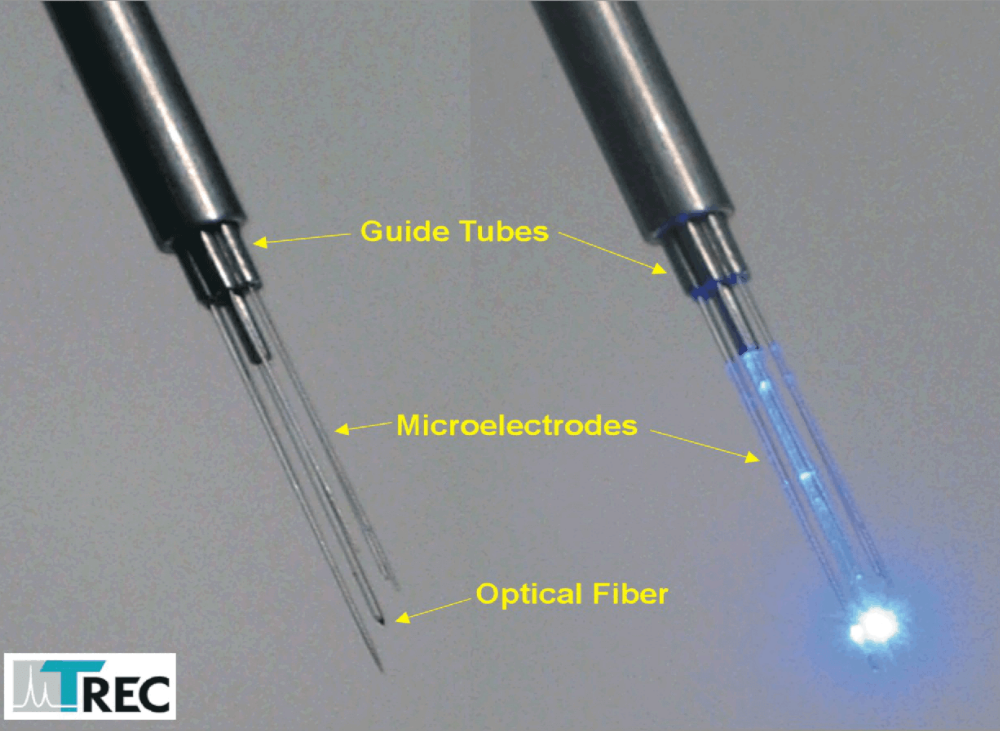
- No electrode connection cables free in air! Complete metal shield around all microelectrodes
- Suitable for cortical and deep brain recordings
- Very close electrode spacing available (down to 80μm)
- Different electrode arrangements available (linear, concentric, etc.)
- Integrated low noise preamplifiers


















































































































| Microdrive Technical Data | |
| Number of motors: | 7 |
| Electrode travel distance per channel: | up to 35.000µm |
| Resolution per step: | 1µm |
| Max. electrode speed: | up to 250µm/s |
| Electrode driving principle: | patented Thomas rubber tube drive |
| Motor control: | via included software and microprocessor motor control unit |
| Pre-Amplifier Technical Data | |
| Number of channels: | 7 |
| Recording bandwidth: | 0.034Hz…25kHz |
| Gain per channels: | x19 |
| Pre-Amplifier input: | AC coupled |
Preamplifier supply voltage: | +/-6V DC |
Operation modes on each channel: | Recording, Stimulation/Lesion, Impedance Ttest |
Stimulation modes: | optical, electrical |
| Further Data | |
| Size: | 158mm x 70mm x 100mm |
| Weight: | 1.6kg |
With this fiber-electrode manipulator a broad range of even very thin shafted probes can be handled, including fiber and wire-electrodes with shaft diameters down to about 25 µm. The patented driving principle of the Eckhorn Manipulator offers an outstanding electrode positioning accuracy. Compared with hydraulic, direct motor driven or cable controlled microdrives, the patented fiber electrode manipulator “System Eckhorn” does not cause hysteresis errors of the electrode movement. Hysteresis error is generally a result of stiction and free motion of the driving mechanism. Our system has a higher degree of positioning accuracy due to its rubber tube driving mechanism being almost absolutely free of stiction and free motion.
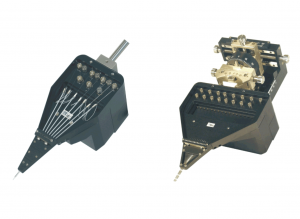


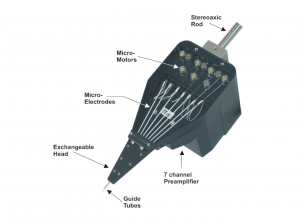


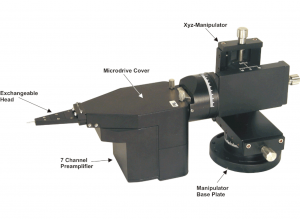


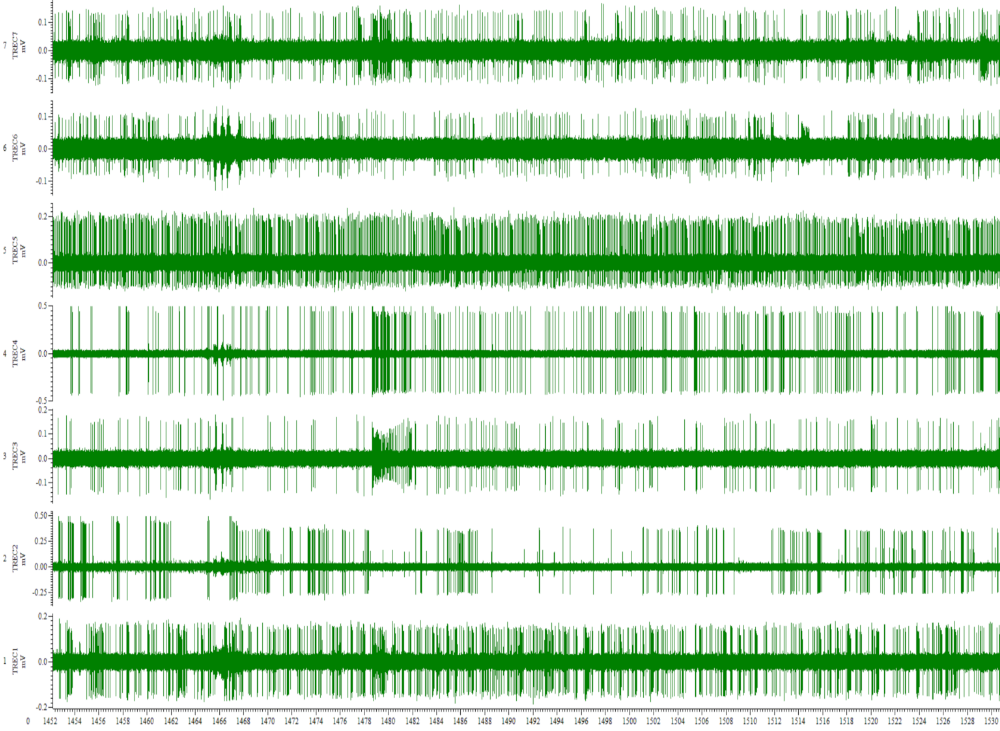
Using Thomas Eckhorn Matrix systems with patented rubber tube drive allows to record neural activity while the electrode is moving in the brain. So it is very easy to search active neurons in the brain.
The Thomas Eckhorn Matrix systems have some advantages in comparison to other multielectrode microdrive systems for neurophysiological research presently available on the market:
- Vibration-free positioning of up to 16 quartz-platinum/tungsten microelectrodes in 1µm steps
- Patented rubber tube driving principle
- High positioning accuracy, due to lack of stiction and free motion
- Easy exchange and calibration of microelectrodes within minutes
- Advances up to 16 microelectrodes/tetrodes independently in 1µm steps up to 35.000µm (other electrode travel distances on request!)
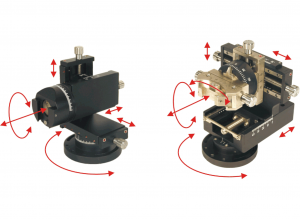
- Xyz-manipulator travel-distance: z=0-30mm, x= ±10mm, y=±10mm
- Continuous low noise recording while the electrode scans the tissue at low velocity,
- No noise introduction in the recordings so that you can search active neurons in the brain
- No electrode connection cables free in air, no electrical noise pickup from environment (e.g. motors, radio stations)! Competitor drives pickup electrical interference from the environment so that you for example can hear radio station music or electrical artifacts from lab devices from the audio monitor loudspeaker instead of neural spikes!
- Electrode moving velocity adjustable (1-250µm/s), moving direction selectable
- Low noise 7 or 16 channel preamplifier is integrated into the microdrive chassis, system is completely shielded to avoid electrical noise pickup from the environment, three preamplifier operation modes: record, electrode impedance test, electrical stimulation/lesioning
- System is completely delivered with motor control device, integrated preamplifier, software, xyz-manipulator, chamber holder, exchangeable head, handheld remote control, etc.
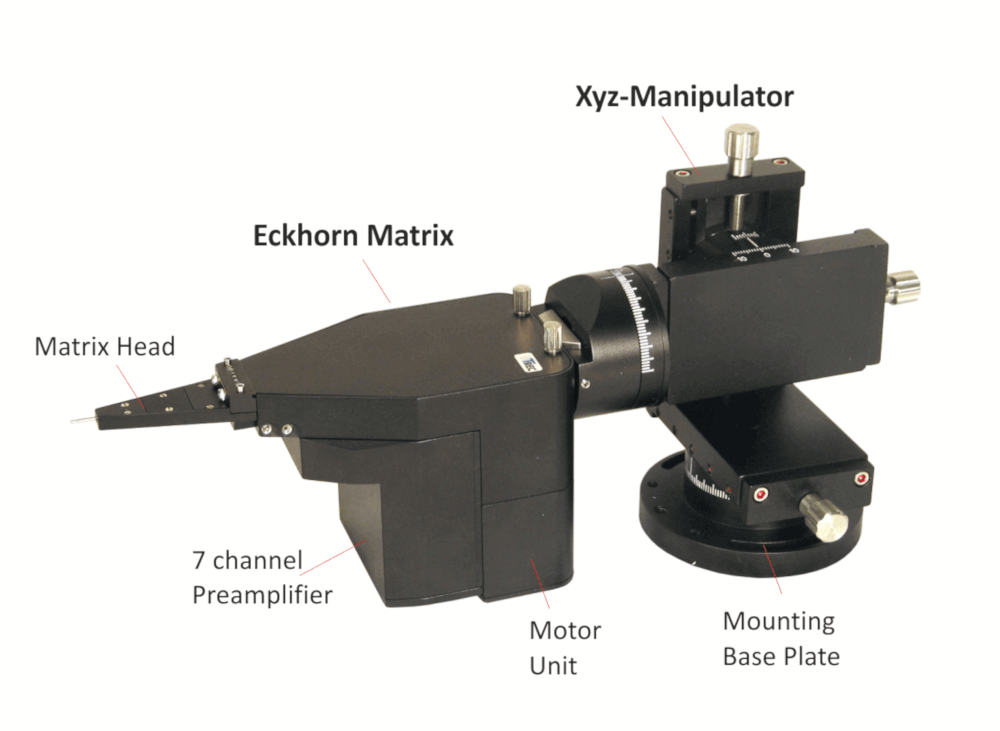
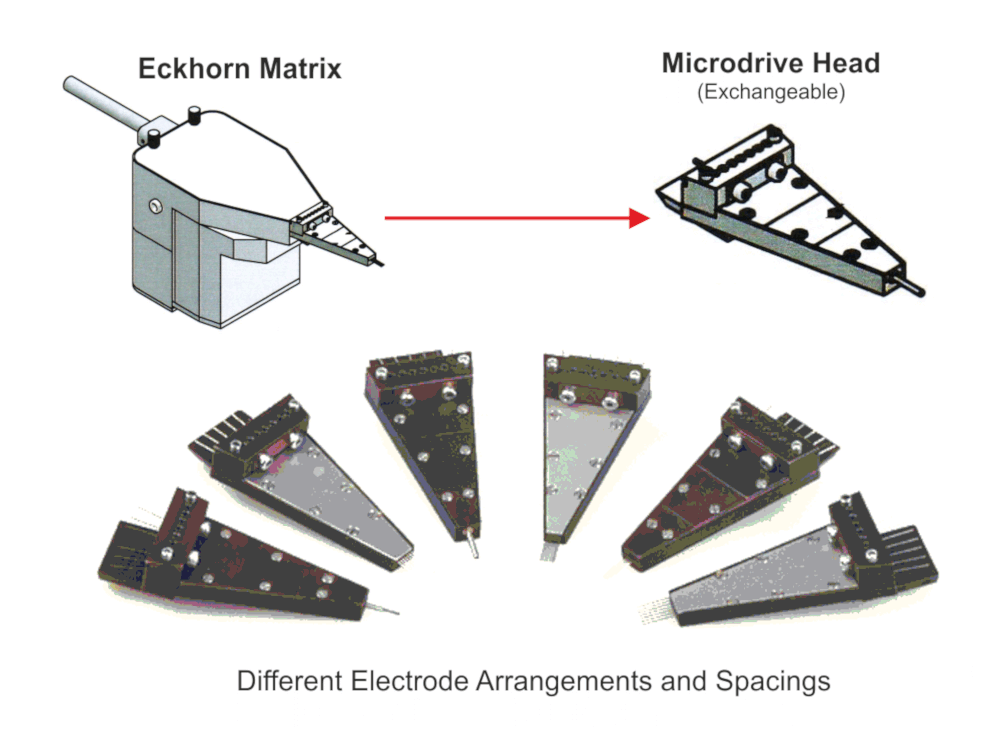
- Exchangeable manipulator head allows manifold electrode configurations and interelectrode spacings
- Electrode spacings from 80µm up to some millimeters possible due to exchangeable Eckhorn Matrix heads
- The system is modular and adaptable to the end user´s requirements.
- The Eckhorn Matrix is usable for cortical as well as deep brain recordings in all kinds of research applications
- User friendly computer controlled system with LAN (local area network) communication via TCP/IP protocol between motor control unit and personal computer. Software package is part of the system.
- Using very thin shaft Thomas microelectrodes (outer diameter 80µm) causes minimal tissue damage.
- Different neurophysiological methods are available for the Eckhorn Matrix systems (e.g. optogenetics, microinjection)


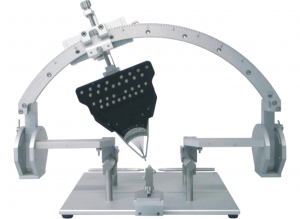
The Eckhorn Matrix system is available for small animal stereotaxic instruments (see figure 10) as well as for primate experiments (see figure 11).


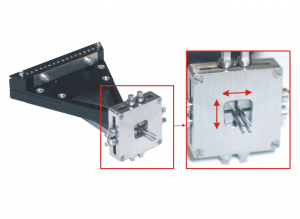
Exchangeable Heads


Thomas RECORDING offers a broad range of exchangeable Microdrive heads with different guide tube arrangements and electrode spacings for the 7- and 16-Electrode Eckhorn Systems.


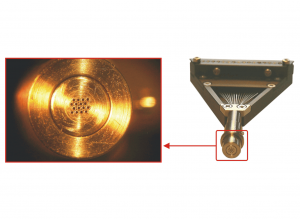


There is a broad range of different Microdrive heads available. We also can offer customized solutions!
Electrical Stimulation / Impedance Test with the Eckhorn Matrix
The Eckhorn Matrix preamplifier is integrated in the microdrive chassis and has three operation modes: Recording / Electrode Impedance Test /Stimulation or Lesioning. The standard operation mode of the preamplifier is “Recording”. To use the other two modes Thomas RECORDING offers a special designed device that is called Mode Selection & Impedance Test Device (MSD).
The MSD is optional available, for more information please see the productpage of MSD.



Thomas RECORDING offers a broad range of solutions for mounting the Eckhorn multielectrode systems to stereotaxic instruments. Below you can find some examples for Eckhorn microdrives mounted to small (see fig. 10) and large animal stereotaxic instruments (see figure 11). It is our strength to adapt our Microdrive to stereotaxic setups. Please feel free to ask for your individual adaptation.





For making drug injection experiments with the Thomas Eckhorn Matrix system we offer a special designed high pressure microinjection system. One of the 7- or 16-single microelectrodes of the Eckhorn Matrix can be replaced by a microinjection cannula. The microinjection cannula (OD=120µm) has a tapered tip and is equipped with the patented Thomas rubber tube drive so that you can position the microinjection cannula tip together with the recording microelectrodes with high positioning accuracy (better than 1µm). The microinjection system consists of a special designed and computer controlled syringe pump that is connected to the microinjection cannula via a special thick wall tubing.
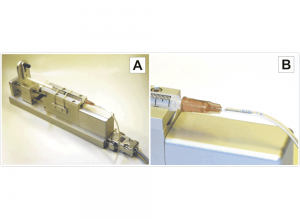


The precision micro motor of the microinjection pump is controlled by the same motor control unit and graphical user interface as the Thomas Eckhorn Matrix. This guarantees a high precision and convenient experimental control of the injection/recording experiment.
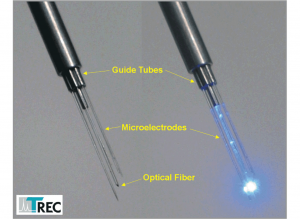
Thomas RECORDING offers a complete range of Eckhorn Matrix accessories for optogenetic experiments. One of the 7- or 16 single microelectrodes or tetrodes is replaced by an optical fiber (OD=120µm). The optical fiber has a tapered tip and is equipped with the patented Thomas rubber tube drive so that you can position the optical fiber tip together with the recording microelectrodes with high positioning accuracy (better than 1µm). Figure 13 shows an Eckhorn Matrix head with concentric guide tube arrangement and a single optical fiber in the center position. The recording microelectrodes are concentrically arranged around the optical fiber in the center of this arrangement.


Downloads
Selected Publications
[27] Mendoza G., Méndez J. C., Pérez O., Prado L., Merchant H. Neural basis for categorical boundaries in the primate pre-SMA during relative categorization of time intervals. Nature communications 9, Article number: 1098 (2018), March 2018, DOI: 10.1038/s41467-018-03482-8
[26] Hasse J. M., Briggs F. Corticogeniculate feedback sharpens the temporal precision and spatial resolution of visual signals in the ferret. PNAS 114 (30) E6222-6230, July 2017, DOI: 10.1073/pnas.1704524114
[25] Merchant H., Averbeck B. B. The Computational and Neural Basis of Rhythmic Timing in Medial Premotor Cortex The Journal of Neuroscience, 31(17):4552-4564, DOI 10.1523/JNEUROSCI.0367-17.2017, 2017
[24] Wolfgang Kruse, Martin Krause, Janna Aarse, Melanie D. Mark, Denise Manahan-Vaughan, Stefan Herlitze Optogenetic Modulation and Multi-Electrode Analysis of Cerebellar Networks In Vivo. Plos ONE, August 2014
[23] Mountcastle V.B., Reitboeck H.J., Poggio G.F., Steinmetz M.A. Adaptation of the Reitboeck method of multiple microelectrode recording to the neocortex of the waking monkey. Journal of Neuroscience Methods, 36 (1991) 77-84
[22] Eckhorn R., Frien A., Bauer R., Woelbern T., Kehr H. High frequency oscillations (60-90Hz) in primary visual cortex of awake monkey. NeuroReport, 4: 243-246, 1993
[21] Eckhorn R., Obermueller A. Single neurons are differently involved in stimulus-specific oscillations in cat visual cortex. Exp. Brain Res., 1993
[20] Eckhorn R., Thomas U. A new method for the insertion of multiple microprobes into neural and muscular tissue, including fiber electrdoes, fine wires, needles and microsensors. Journal of Neuroscience Methods, 49 (1993) 175-179
[19] Baker S.N. and Lemon R.N. Precise Spatiotemporal Repeating Patterns in Monkey Primary and Supplementary Motor Areas Occur at Chance Levels. J Neurophysiol, Oct 2000; 84: 1770 – 1780
[18] Reinagel P., Reid R.C. Temporal Coding of Visual Information in the Thalamus. Journal of Neuroscience, July 15, 2000, 20(14):5391-5400
[17] Battaglia-Mayer A., Ferraina S., Genovesio A., Marconi B., SquatritoS., Molinari M., Lacquaniti F., Caminiti R. Eye-Hand Coordination during Reaching. II. An Analysis of the Relationships between Visuomanual Signals in Parietal Cortex and Parieto-frontal Association Projections. Cerebral Cortex Jun 2001;11:528-544; 1047-3211/01
[16] Baker S.N., Spinks R., Jackson A., Lemon R.N. Synchronization in Monkey Motor Cortex During a Precision Grip Task. I. Task-Dependent Modulation in Single-Unit Synchrony. J Neurophysiol, Feb 2001; 85: 869 – 885.
[15] Lee D. Analysis of phase-locked oscillations in multi-channel single-unit spike activity with wavelet cross-spectrum. Journal of Neuroscience Methods 115 (2002) 67-75
[14] Bruno R.M. and Simons D.J. Feedforward Mechanisms of Excitatory and Inhibitory Cortical Receptive Fields. Journal of Neuroscience, December 15, 2002, 22(24):10966-10975
[13] Miller L.E., Holdefer R.N., Houk J.C. The Role of the Cerebellum in Modulating Voluntary Limb Movement Commands. Archives Italiennes de Biologie (2002) 140: 175-182
[12] Kruse W., Hoffmann K.-P. Fast gamma oscillations in areas MT and MST occur during visual stimulation, but not during visually guided manual tracking. Exp Brain Res (2002) 147:360-373
[11] Gail A., Brinksmeyer H.J., Eckhorn R. Simultaneous mapping of binocular and monocular receptive fields in awake monkeys for calibrating eye alignment in a dichoptical setup. Journal of Neuroscience Methods 126 (2003) 41 – 56
[10] Kwegyir-Afful E.E., Keller A. Response Properties of Whisker-Related Neurons in Rat Second Somatosensory Cortex. J Neurophysiol 92: 2083-2092, 2004
[9] Crowe D.A., Chafee M.V., Averbeck B.B., Georgopoulos A.P. Participation of primary motor cortical neurons in a distributed network during maze solution: representation of spatial parameters and time-course comparison with parietal area 7a. Exp Brain Res (2004) 158: 28-34
[8] Naselaris T., Merchant H., Amirikian B., Georgopoulos A.P. Spatial Reconstruction of Trajectories of an Array of Recording Microelectrodes. J Neurophysiol, Vol 93, 2318-2330, 2005
[7] Averbeck B.B., Chafee M.V., Crowe D.A., Georgopoulos A.P. Parietal Representation of Hand Velocity in a Copy Task. J Neurophysiol, Vol 93, 508-518, 2005
[6] Averbeck B.B., Sohn J.-W., Lee D. Activity in prefrontal cortex during dynamic selection of action sequences. Nature Neuroscience 9, 276-282 (01 Feb 2006)
[5] Anderson B., Harrison M., Sheinberg D.L. A multielectrode study of the inferotemporal cortex in the monkey: effects of grouping on spike rates and synchrony. Neuroreport 2006, 17(4):407-411
[4] Gothard K.M., Battaglia F.P., Erickson C.A., Spitler K.M., Amaral D.G. Neural Responses to Facial Expression and Face Identity in the Monkey Amygdala. Journal of Neurophysiology 97:1671-1683, 2007
[3] A. Thiele, K.-P. Hoffmann Neuronal firing rate, inter-neuron correlation and synchrony in area MT are correlated with directional choices during stimulus and reward expectation. Experimental Brain Research (2008) 188:559-577
[2] Laurens W. J. Bosman, Sebastiaan K. E. Koekkoek, Joël Shapiro, Bianca F. M. Rijken, Froukje Zandstra, Barry van der Ende, Culen B. Owens, Jan-Willem Potters, Jornt R. de Gruijl, Tom J. H. Ruigrok, Chris I. De Zeeuw Encoding of whisker input by cerebellar Purkinje cells. Journal of Physiology 588.19 (2010): 3757-3783
[1] Ying Cao, Selva K. Maran, Mukesh Dhamala, Dieter Jaeger, Detlef H. Heck Behavior related pauses in simple spike activity of mouse Purkinje cells are linked to spike rate modulation. Journal of Neuroscience 2012 June 20; 32(25): 8678-8685
(ELECTROCHEMICAL PRODUCTS)
Microelectrodes > Electrode Pol. Medical Products Neuroscience Products
Electrochemical Single-core Microelectrodes
Microelectrodes > Electrode Polishing Tools Medical Products Neuroscience Products
Electrochemical Single-core Microelectrodes
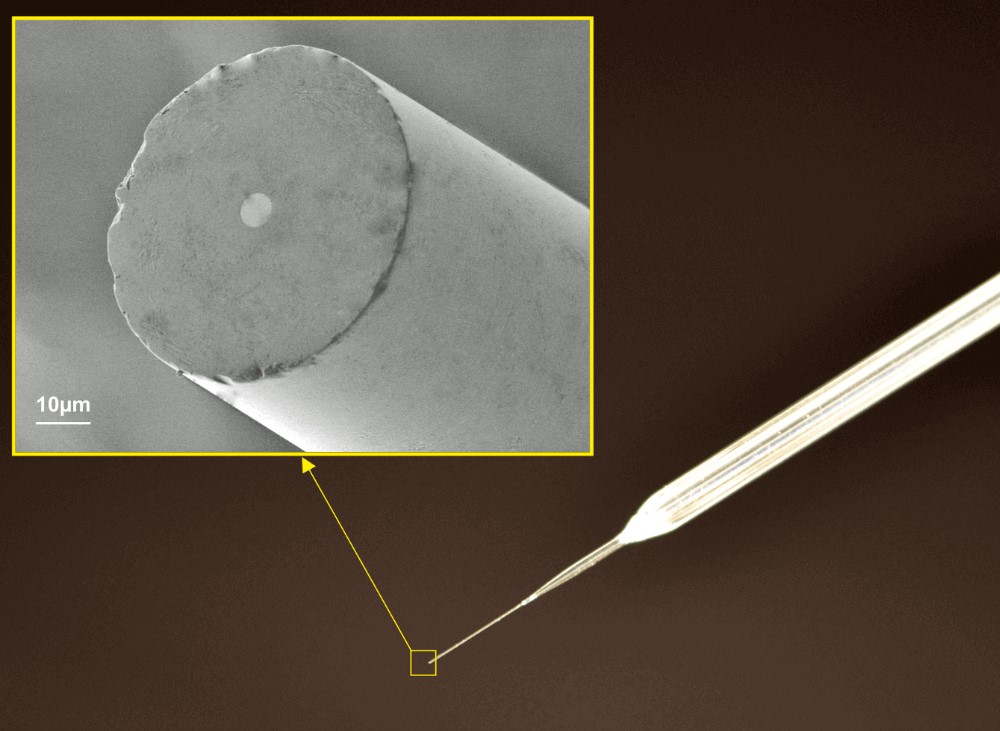
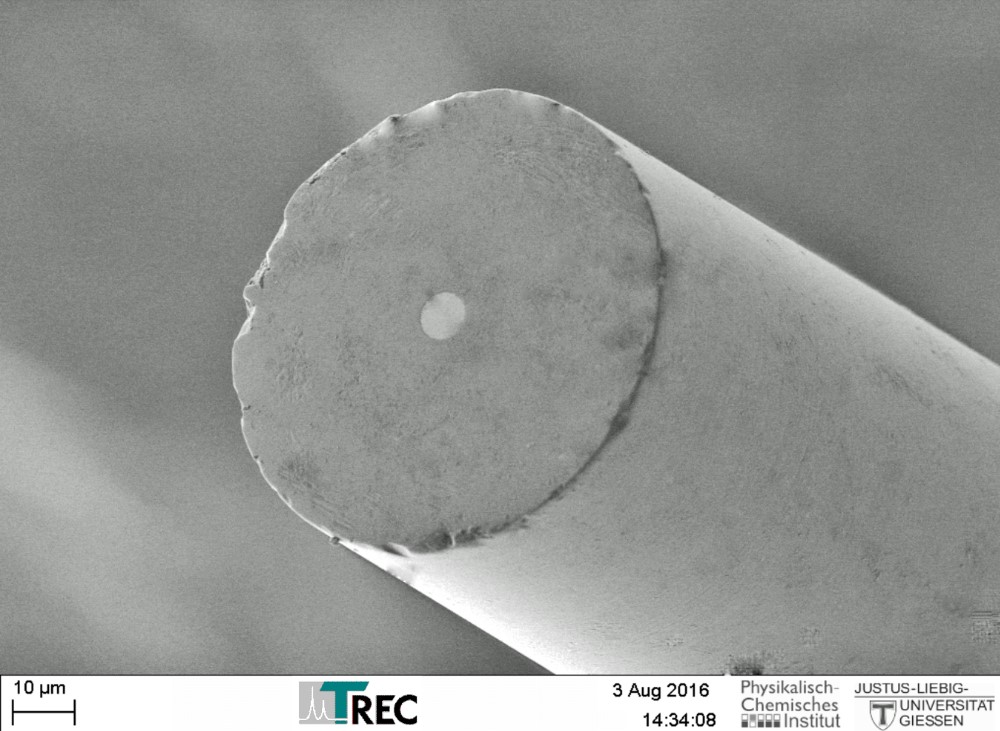
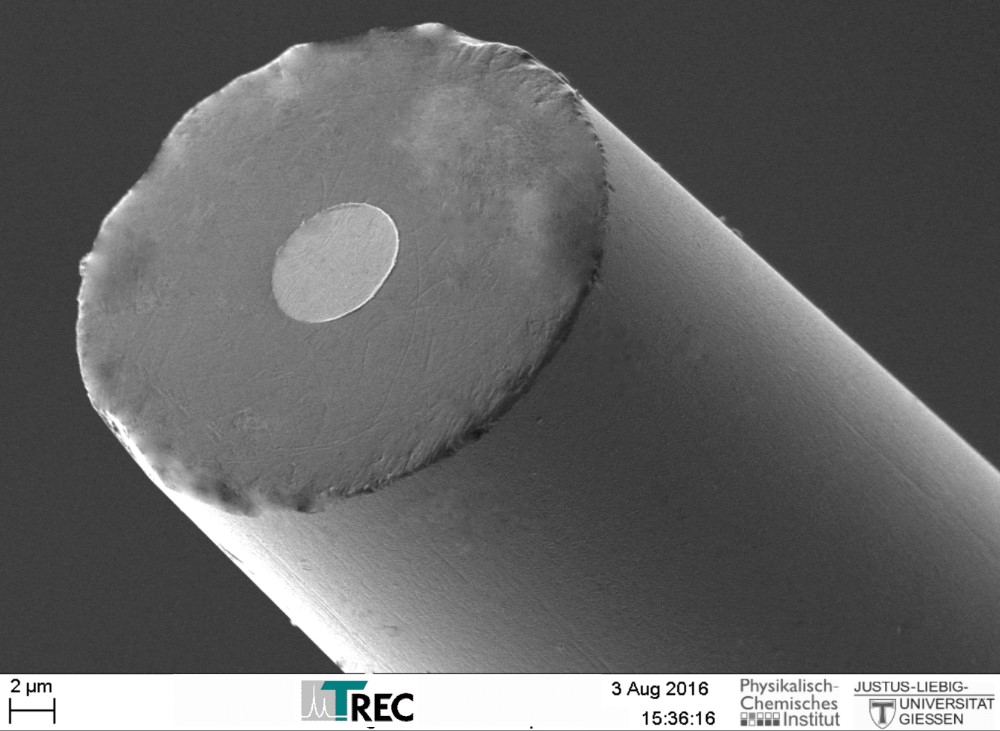
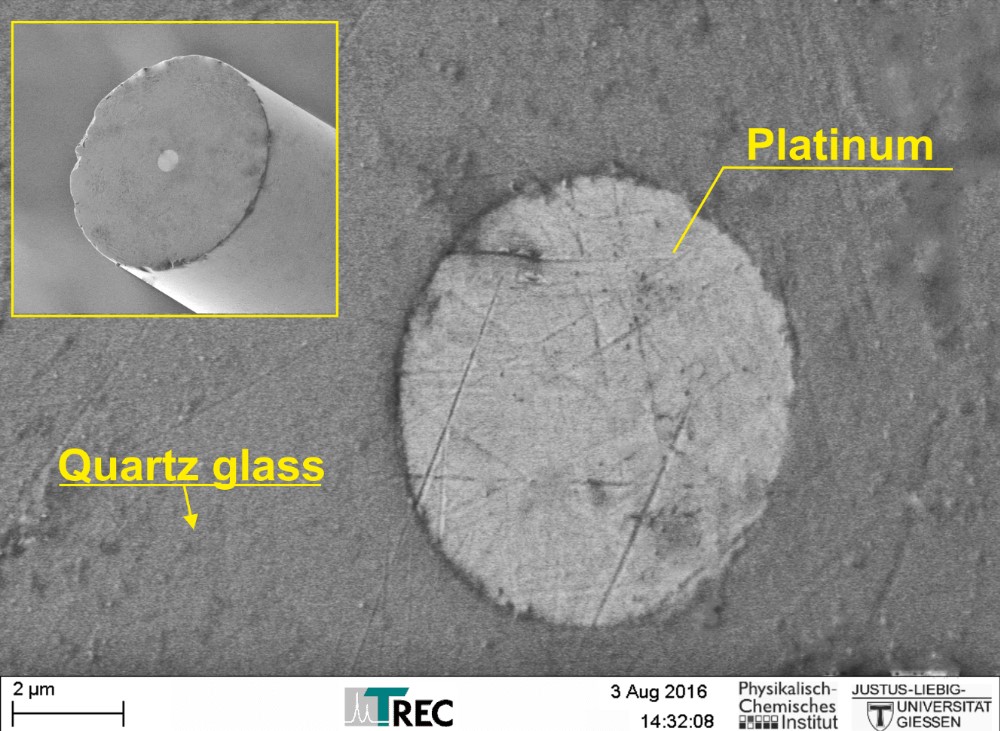
Thomas RECORDING electrochemical disc microelectrodes were originally designed for SECM (Scanning ElectroChemical Microscopy), but they are also suitable as working electrodes for other electrochemical applications like voltammetry or amperometry.
The electrodes are based on unique single metal core fibers of platinum or platinum/tungsten alloy, insulated with quartz glass.
Key features:
- Material: quartz glass insulated platinum or platinum/tungsten
- Unique manufacturing technique offers high reproducibility of tip geometry
- Tip geometry: highly centered metal core
- Signal quality: very good signal to noise ratio
- Quartz glass offers better electrical characteristics as borosolicate glass
- Tip shape: different tip shapes avaible with tip diameters in the µm range























































































| Core conductor material: | platinum (95%), tungsten (5%) |
| Insulation material: | quartz glass |
| Number of available channels: | 1-channel electrochemical electrode (4-channel or 7-channel available) |
| Tip shape: | (B) pulled & ground, disc type (only available for 4 channel electrochemical electrode) (C) only ground, disc type (most common) |
| Connectors: | gold plated male pin, 0.8mm |
Matching female connector available from
Thomas RECORDING
Dimensions:
L1: Standard is 80mm, custom adaption possible
L2: Standard is 5mm, custom adaption possible
L3: Standard is 40mm, custom adaption possible
d1: 2.0mm
See Figure 7 for the dimensions of the electochemical multicore electrode.
Custom electochemical electrodes are available on request.
Features
A special manufacturing process guarantees a highly centered metal core within the glass insulation and a high reproducibility of the RG ratio.
Every electrode produced by Thomas RECORDING is microscopically controlled and tested by cyclic voltammetry. The test results for each electrode are documented by test certificates enclosed to your shipment. The electrochemical active diameter is determined for every individual electrode by measuring the diffusion limiting current. For more information please be referred to the product information sheet below, or send us your request.



RELATED PUBLICATIONS
[7] Etienne M., Moulin J-P., Gourhand S. Accurate control of the electrode shape for high resolution shearforce regulated SECM. Electrochimica Acta; 2013; Volume 110, pp 16-21; DOI: 10.1016./j.electacta.2013.03.096
[6] Etienne M., Dossot M., Grausem J., Herzog G. Combined Raman Microspectrometer and Shearforce Regulated SECM for Corrosion and Self-Healing Analysis. Analytical Chemistry; 2014; 86 (22), pp 11203-11210; DOI: 10.1021/ac502670t
[5] Etienne M., Lhenry S., Cornut R., Lefrou C. Optimization of the shearforce signal for scanning electrochemical microscopy and application for kinetic analysis. Electrochimica Acta; 2013; Volume 88: pp 877-884, DOI:10.1016/j.electacta.2012.09.063
[4] Etienne M., Layoussifi B., Giornelli T. SECM-based automate equipped with a shearforce detection for the characterization of large and complex samples. Electrochemisty Communications, 2012, Volume 15, Issue 1, pp 70-73, DOI: 10.1016/j.elecom.2011.11.028
[3] van Megen M.J.J., Odijk M., Wiedemair J., Olthuis W., van den Berg A. Differential cyclic voltammetry for selective and amplified detection. Journal of Electroanalytical Chemistry, Volume 681 Page 6-10, 2012
[2] Etienne M., Layoussifi B., Giornelli Th., Jacquet D. SECM-based automate equipped with a shearforce detection for the characterization of large and complex samples. Electrochemical Communications; 2011; 15: 70-3
[1] Cornut R., Bhasin A., Lhenry S., Ethienne M., Lefrou Ch. Accurate and Simplified Consideration of the Probe Geometrical Defaults in Scanning Electrochemical Microscopy: Theoretical and Experimental Investigations. Analytical Chemistry, 2011; 83: 9669-75
(SONSTIGE ELEMENTE)
Questions? Please don’t hesitate contacting us!



NEWS


PRODUCTS



SOLUTIONS



DISTRIBUTORS
SIGN UP TO OUR NEWSLETTER